Translation of Text on PDMS Membranes in Microfluidic Technology
2025/10/28
0
As an emerging interdisciplinary technology, microfluidic technology features core advantages of “miniaturization, integration, and low consumption” and demonstrates enormous potential in fields such as biomedical testing, environmental monitoring, and chemical synthesis. Material selection is critical to the design and performance realization of microfluidic chips. Among various materials, polydimethylsiloxane (PDMS) membranes, with their unique physicochemical properties, have become one of the indispensable core materials in microfluidic systems. Their application scenarios cover multiple key links including fluid control, biochemical analysis, and cell culture.
PDMS Membranes in Microfluidic Technology
I. Core Properties of PDMS Membranes as Ideal Materials for Microfluidics
The wide application of PDMS membranes in the microfluidic field stems from their “all-round” properties that naturally meet the requirements of microfluidic systems. These properties directly determine their functional advantages in different application scenarios:
-
High Gas Permeability: PDMS membranes exhibit excellent permeability to gases such as oxygen and carbon dioxide (the oxygen transmission rate is approximately 1.5×10⁻¹⁰ cm³・cm/(cm²・s・Pa)). Moreover, the gas transmission rate can be precisely adjusted by controlling the membrane thickness. Gas exchange in microchannels can be achieved without additional gas circuit design, which is crucial for scenarios such as cell culture and anaerobic reactions.
-
Good Flexibility and Elasticity: PDMS membranes have an extremely low elastic modulus (about 1-3 MPa) and can withstand repeated stretching and compression without breaking. They not only enable dynamic control of microvalves and micropumps through external force driving but also adapt to the bending and folding requirements of flexible microfluidic chips.
-
Excellent Biocompatibility: PDMS itself is non-toxic and non-irritating. After plasma treatment or chemical modification, its surface can adsorb proteins and immobilize biomolecules (such as antibodies and enzymes) without significantly inhibiting cell activity, thus meeting the safety requirements for processing biological samples (e.g., blood and cells).
-
Optical Transparency: PDMS membranes have a light transmittance of over 90% in the wavelength range of 250-1200 nm. They can be directly used with optical detection technologies (such as fluorescence imaging and UV-visible spectrophotometry) to realize real-time visual monitoring of reaction processes in microchannels.
-
Ease of Processing: PDMS membranes can be fabricated into structures of different thicknesses (from several micrometers to hundreds of micrometers) and different patterns (such as micropore arrays and microchannels) through simple processes like casting, molding, and cutting. Additionally, they can be sealed with glass, silicon wafers, and other polymers (e.g., PMMA) via plasma bonding, reducing the manufacturing cost and difficulty of microfluidic chips.
II. Core Application Scenarios of PDMS Membranes in Microfluidics
Based on the aforementioned properties, PDMS membranes act as “functional carriers” in microfluidic systems. Their applications can be divided into the following four core fields, each with mature technical solutions:
(1) Fluid Control: Core Driving Components of Microvalves and Micropumps
One of the core requirements of microfluidic systems is to achieve precise control of microscale fluids (from nanoliters to microliters). The flexibility of PDMS membranes makes them ideal “valve cores” or “pump membranes” for microvalves and micropumps:
-
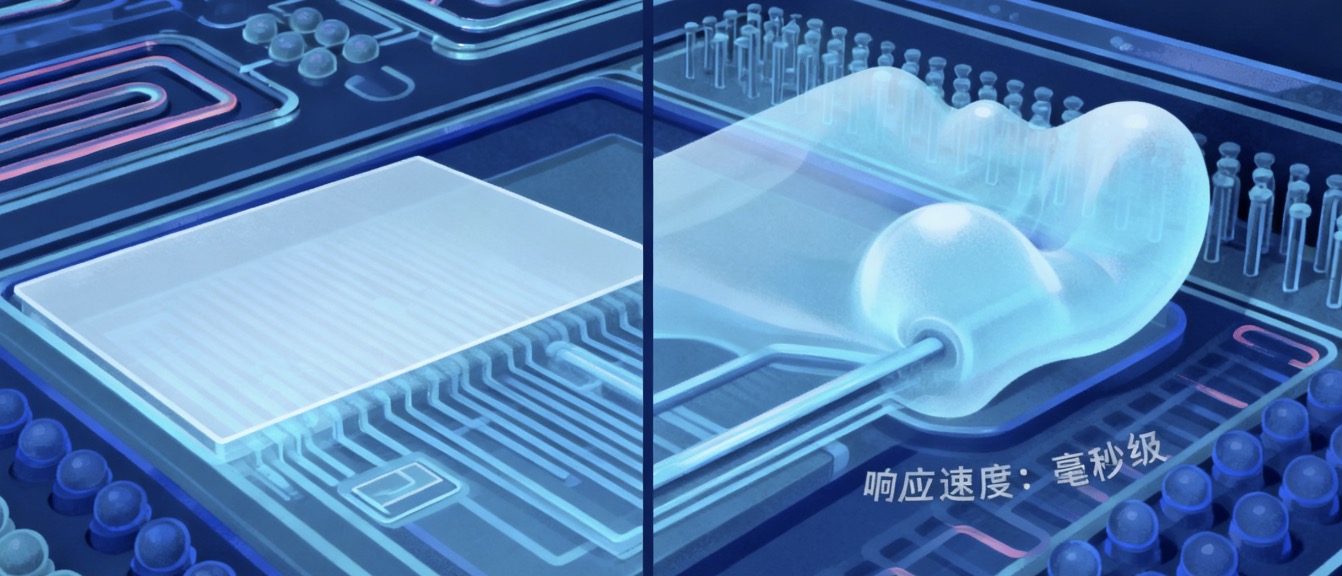
PDMS Membrane Microvalves: A closed “control chamber” is designed beside the microchannel, with the top of the chamber separated from the channel by a PDMS membrane. When air pressure (or hydraulic pressure) is applied to the control chamber, the PDMS membrane bulges toward the channel, squeezing the channel to achieve “closure”; when the pressure is removed, the membrane returns to its original shape, and the channel is re-“opened”. These valves have the advantages of fast response speed (millisecond level), good sealing performance (leakage rate < 10⁻¹² L/min), and reusability (up to tens of thousands of cycles). They are widely used for fluid switching (e.g., sample distribution and reagent mixing) in Lab-on-a-Chip systems. For example, in nucleic acid amplification chips, multiple sets of PDMS membrane microvalves can be used to realize the step-by-step addition of samples, primers, and enzyme reagents, avoiding cross-contamination.
-
PDMS Membrane Micropumps: Based on the principle of “pneumatic driving”, these micropumps achieve one-way fluid delivery either through the orderly opening and closing of three PDMS membrane microvalves (similar to the working mode of peristaltic pumps) or via high-frequency vibration of PDMS membranes driven by piezoelectric sheets to generate negative pressure. Such micropumps do not require external large-scale pumps, with a volume only 1/1000 of that of traditional pumps. Their flow rate can be precisely adjusted within the range of 0.1-100 μL/min, making them suitable for portable microfluidic devices (e.g., supporting pumps for on-site detection test strips).
(2) Biochemical Analysis: Functional Interfaces for Separation, Enrichment, and Detection
In biochemical analysis microfluidic chips, PDMS membranes often serve as “selective permeation” or “reaction carrier” interfaces to realize sample pretreatment and detection:
-
Gas Separation and Detection: Utilizing the selective permeability of PDMS membranes to specific gases, gas detection chips can be constructed. For instance, in environmental monitoring, PDMS membranes are used as the “gas inlet window” of the chip. Volatile organic compounds (VOCs) in the air can penetrate the membrane and enter the chip interior, reacting with sensitive materials (e.g., fluorescent probes). Qualitative and quantitative detection of VOCs is achieved through changes in optical signals. In medical testing, oxygen in blood can be separated via PDMS membranes to realize real-time monitoring of blood gas indicators (e.g., blood oxygen saturation).
-
Substance Enrichment and Purification: Chemical modification of the PDMS membrane surface (e.g., grafting hydrophilic groups or antibodies) enables specific adsorption of target substances. For example, in protein detection chips, PDMS membranes are made into micropore arrays, with antigens and antibodies immobilized on their surfaces. When the sample flows through, the target proteins are adsorbed and enriched by the membrane, and then purified through elution—greatly improving the sensitivity of subsequent detection (the detection limit can be reduced to the pg/mL level).
-
Enzymatic Reaction Carriers: The porous structure (or surface roughness) of PDMS membranes can increase the enzyme loading capacity. When used as enzyme immobilization carriers, they not only prevent enzyme loss in the fluid but also ensure sufficient contact between substrates and enzymes. For example, in glucose detection chips, glucose oxidase is immobilized on the surface of PDMS membranes. When the glucose solution flows through, hydrogen peroxide produced by the enzymatic reaction can be detected by an electrochemical sensor, enabling rapid quantification of blood glucose (detection time < 1 minute).
(3) Cell Research: Tools for Constructing Biomimetic Microenvironments
Microfluidic technology provides a “biomimetic microenvironment” for cell research, and the biocompatibility and gas permeability of PDMS membranes make them core carriers for cell culture and observation:
-
3D Cell Culture Chips: PDMS membranes are made into “support membranes” with micropores (diameter: 5-10 μm). A “cell chamber” and a “nutrient chamber” are designed on both sides of the membrane, respectively. Cells attach and grow in the cell chamber; nutrients (e.g., components of the culture medium) can penetrate through the micropores of the PDMS membrane to the cell side, while metabolic wastes are discharged in the reverse direction—simulating the “nutrient exchange” microenvironment of cells in vivo. Such chips can be used for in vitro culture of tumor cells to observe their proliferation and migration characteristics, or for anti-cancer drug screening (e.g., controlling the drug concentration gradient through PDMS membranes to study dose effects).
-
Cell Co-Culture Systems: Utilizing the “physical isolation-substance exchange” property of PDMS membranes, co-culture of two types of cells can be realized in the chip. For example, endothelial cells are inoculated on one side of the PDMS membrane, and smooth muscle cells on the other side. The micropores of the membrane allow bidirectional diffusion of cytokines (e.g., VEGF), simulating the intercellular interaction of the vascular wall and facilitating research on the mechanism of angiogenesis.
(4) Biomimetic Microsystems: Simulating the Functions of Human Organs
In the recently developed “Organ-on-a-Chip” technology, PDMS membranes are key materials for simulating the physical structure and functions of human tissues:
-
Lung-on-a-Chip: A PDMS membrane is placed in the microchannel. One side of the membrane is filled with air (simulating the alveolar cavity), and the other side with culture medium containing blood cells (simulating blood vessels). Periodic vibration of the PDMS membrane is driven by external force (simulating breathing movement) to realize transmembrane exchange of gases (e.g., oxygen and carbon dioxide). Meanwhile, the damage of pollutants (e.g., PM2.5) to lung epithelial cells can be observed, which is applicable to drug toxicity testing or environmental hazard assessment.
-
Skin-on-a-Chip: PDMS membranes are used as “dermal support”. Fibroblasts and keratinocytes are inoculated on their surfaces to construct a multi-layer skin model. The gas permeability of the membrane can simulate the “respiratory function” of the skin. Additionally, the permeability of the membrane can be used to test the absorption efficiency of skincare products or evaluate the skin irritation of chemicals, replacing traditional animal experiments.
III. Challenges and Future Development Directions of PDMS Membrane Applications
Although PDMS membranes are widely used in microfluidics, there are still some technical bottlenecks to be addressed:
-
Non-Specific Adsorption Issue: The surface of PDMS membranes is hydrophobic, which easily causes non-specific adsorption of proteins and small-molecule organic substances, leading to sample loss or detection interference. Currently, this problem can be partially solved through surface coatings (e.g., polyethylene glycol, hydrophilic polymers) or plasma modification, but long-term stability still needs to be improved.
-
Control of Membrane Thickness Uniformity: Microfluidic chips have extremely high precision requirements for PDMS membrane thickness (e.g., micrometer level). Traditional molding processes are difficult to achieve large-area uniformity. In the future, more precise preparation technologies (e.g., spin-coating, laser cutting) need to be developed.
-
Insufficient Functional Integration: Existing PDMS membranes mostly undertake a single function (e.g., microvalve, gas exchange). In the future, “multi-material composite” (e.g., combining PDMS membranes with metal nanoparticles and graphene) should be adopted to realize “one membrane with multiple functions” (e.g., simultaneously having fluid control and electrochemical detection functions), further reducing the chip volume.
In the future, as microfluidic technology moves toward “higher integration, intelligence, and portability”, the application of PDMS membranes will show three major trends: First, developing toward “ultra-thinness” (thickness < 1 μm) to improve gas permeability and response speed; second, combining with “smart materials” (e.g., shape-memory polymers, stimuli-responsive materials) to realize self-driven and self-regulating fluid control; third, expanding applications in the field of “personalized medicine”, such as wearable microfluidic devices based on PDMS membranes for real-time monitoring of physiological indicators like blood glucose and sweat electrolytes.
IV. Conclusion
With unique advantages such as gas permeability, flexibility, and biocompatibility, PDMS membranes have become a key material support for microfluidic technology to move from “laboratory research” to “practical application”. Their applications in fluid control, biochemical analysis, cell research, and Organ-on-a-Chip not only promote the performance improvement of microfluidic systems but also provide more efficient and low-cost technical solutions for fields such as biomedicine and environmental science. Although challenges such as non-specific adsorption and processing precision still exist, with the advancement of material modification technologies and microfabrication processes, PDMS membranes will play a more core role in the microfluidic field, facilitating the transformation of “miniaturized laboratories” into “portable and intelligent devices”.